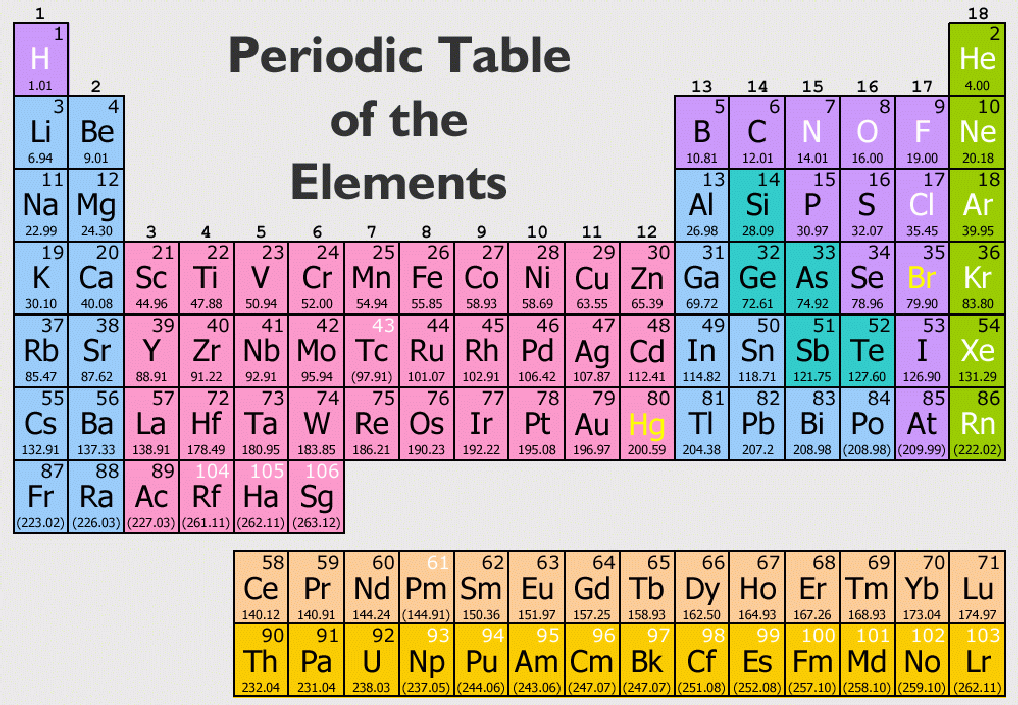
The periodic table is a fundamental tool in the field of chemistry, serving as a roadmap to understanding the elements that make up our universe. With a total of 118 confirmed elements, each with unique properties and applications, the periodic table not only helps scientists but also the general public to comprehend the material world around them. In this article, we will explore the periodic table in-depth, answering the question: how many elements are on the periodic table and why this knowledge is essential.
Our journey will uncover the history of the periodic table, the significance of each element, and how they are organized. Furthermore, we will touch on the implications of these elements in various fields, including medicine, technology, and environmental science. Knowledge of the periodic table is crucial for anyone interested in science, and we aim to provide you with a thorough understanding.
By the end of this article, you will not only know how many elements are on the periodic table, but you will also appreciate the relevance of these elements in everyday life. So, let’s dive into the fascinating world of the periodic table!
Table of Contents
- What is the Periodic Table?
- History of the Periodic Table
- Elements and Their Properties
- How Many Elements Are on the Periodic Table?
- Classification of Elements
- The Significance of the Periodic Table
- Future of the Periodic Table
- Conclusion
What is the Periodic Table?
The periodic table is a systematic arrangement of chemical elements, organized by their atomic number, electron configurations, and recurring chemical properties. It serves as an essential tool for chemists and scientists, allowing them to predict the characteristics and behaviors of elements based on their position in the table.
History of the Periodic Table
The journey of the periodic table began in the early 19th century with the work of various scientists. Dmitri Mendeleev, a Russian chemist, is often credited with its creation in 1869. Mendeleev arranged the elements according to their atomic mass and noticed that certain properties occurred at regular intervals, which led to the development of the periodic law. This arrangement allowed Mendeleev to predict the existence of undiscovered elements, showcasing the table's predictive power.
Throughout the years, the periodic table has undergone several revisions and expansions, particularly with the discovery of new elements and advances in atomic theory. Today, the periodic table consists of 118 confirmed elements, with the latest additions being copernicium, nihonium, moscovium, livermorium, tennessine, and oganesson.
Elements and Their Properties
Each element on the periodic table has its own unique set of properties, including atomic number, atomic mass, and electron configuration. Here is a brief overview of some key properties of elements:
- Atomic Number: The number of protons in the nucleus of an atom, which determines the element's identity.
- Atomic Mass: The weighted average mass of an element's isotopes.
- Electron Configuration: The distribution of electrons in an atom's orbitals, which influences its chemical behavior.
- State of Matter: Elements can exist as solids, liquids, or gases at room temperature.
How Many Elements Are on the Periodic Table?
As of now, there are 118 confirmed elements on the periodic table. These elements range from hydrogen (the lightest) to oganesson (the heaviest). The elements are categorized into metals, nonmetals, and metalloids, each exhibiting distinct characteristics.
New Elements and Discoveries
New elements are continually being researched and synthesized in laboratories around the world. The process of discovering new elements involves high-energy collisions of atomic nuclei, resulting in the formation of heavier elements that often have very short half-lives. As a result, the periodic table may expand in the future, adding even more elements to its collection.
Classification of Elements
The periodic table is organized into several categories that help in understanding the properties of elements. The main classifications include:
- Metals: Typically good conductors of heat and electricity, malleable, and ductile.
- Nonmetals: Poor conductors, often brittle in solid form, and can exist in various states (solid, liquid, gas).
- Metalloids: Elements that have properties intermediate between metals and nonmetals.
Groups and Periods
The periodic table consists of columns (groups) and rows (periods). Elements in the same group often share similar chemical properties. For example, elements in Group 1 (alkali metals) are highly reactive with water, while elements in Group 18 (noble gases) are known for their lack of reactivity.
The Significance of the Periodic Table
The periodic table is not just a collection of elements; it is a vital tool in various fields including chemistry, physics, biology, and engineering. Understanding the properties of elements allows scientists and researchers to:
- Predict chemical reactions and compounds.
- Develop new materials with specific properties for technological advancements.
- Investigate the behavior of elements in biological systems.
Future of the Periodic Table
The future of the periodic table is promising, with ongoing research aimed at discovering new elements and understanding the properties of existing ones. Advanced technologies, such as particle accelerators, are enhancing our ability to study the atomic and subatomic levels, potentially leading to the synthesis of new elements.
Moreover, as our understanding of chemistry and atomic theory evolves, the periodic table may undergo further revisions to accommodate new discoveries and insights.
Conclusion
In conclusion, the periodic table is a critical component of scientific understanding, containing a total of 118 elements that play essential roles in various fields. From the historical development of the table to its future potential, the knowledge of how many elements are on the periodic table is not just academic; it impacts our everyday lives and the advancement of technology.
We encourage you to share your thoughts in the comments below, explore related articles, or delve deeper into the captivating world of chemistry!
References
- American Chemical Society. (2021). The Periodic Table: An Overview.
- Royal Society of Chemistry. (2020). Periodic Table of Elements.
- NASA. (2019). Periodic Table of Elements: A NASA Perspective.
You Might Also Like
Understanding Kindle File Formats: A Comprehensive GuideDiscovering Police Auctions Near Me: A Comprehensive Guide
Understanding Escort Sites: A Comprehensive Guide
Fresh Juice: The Ultimate Guide To Healthy Living
How To Get Rid Of Moths: Effective Strategies For A Moth-Free Home
Article Recommendations
- Nina Aoulik
- Deacon Johnson
- Efficient Strategies_0.xml
- Legal Seafood Recipes Crab Cakes
- Chevy S10 Steering Wheel
- Michael Jordan Tequila Reposado
- How Did Jennifer Syme Pass Away
- Career Advancement_0.xml
- Corinne Foxx
- Water Softener Overflowing Brine Tank